Mining
The Rush For Jade in British Columbia







The Rush For Jade in British Columbia
Infographic presented by Electra Stone
Many years ago, jade used to travel to China along the Silk Road. Today, more and more jade is being shipped across the Pacific Ocean from British Columbia.
China is the epicenter of jade demand and culture, and today’s infographic shows how jade is formed, the rush for BC jade, and how jade gets from the boulder to the market.
How Jade is Formed
Finding high-quality jade is extremely difficult, which is part of the reason it is so valuable. Jade is created in areas of the world that have subduction zones, such as around the Ring of Fire.
Subduction occurs when two tectonic plates collide and one is forced under the other. The elements are carried deep into the earth, where immense heat and pressure creates the necessary conditions for the formation of jade. This process takes 60 million years.
Most of the world’s jade forms in areas with this kind of intense geological activity. Xinjiang, home to China’s most important nephrite jade deposits, his near where the Indian plate is colliding with the Eurasian plate.
In British Columbia, the conditions are similar, and jade can now be found throughout Canada’s westernmost province. Specifically, there are three areas where today jade is mined: Dease Lake, Mount Ogden, and Cassiar.
The Jade Market
The global market for jade is dominated by Myanmar (formerly Burma) where the majority of jadeite is produced. The size of the reported jade market is largely dependent on data from Myanmar. Before conflict and mine shutdowns resumed in the country in 2011, jade sales were estimated to be $3.5 billion per year.
These shutdowns led to a supply gap in jade, and that is where British Columbia comes in: the production of BC jade has increased from 1.7% to 8.3% from 2011 to 2014.
A 2013 Harvard report put out a more in-depth assessment of the global jade market, and estimated it to be $8 billion.
The BC Jade Market is Booming
Demand for the most desired jade, which comes in an emperor green colour, has put significant price pressure on BC jade. Production of BC jade has doubled, and prices for gem quality jade have jumped to $200-$1,000 per kg.
From Source to Market
BC jade can be found in hard rock deposits or in alluvial boulders that have been moved by glaciers over time.
“New mine jade” refers to jade found in hard rock deposits. This is typically more weathered and more susceptible to cracking.
“Old mine jade” is jade that has been dragged by glaciers in boulder form. Only the best boulders survive, and typically these are of high quality.
Jade is similar to gold in that it can be found in a pure form in nuggets in streams and rivers. Often, the most ambitious Chinese buyers may fly in via helicopter to the northern jade sites to buy jade “off the bucket” in cash. This ensures the best quality jade and miners also benefit.
The jade then typically makes its way to a hub like Vancouver to get shipped overseas to China. China is by far the world’s largest market for jade, where it is considered a hard asset and a symbol of wealth, purity, and spirituality. China is also home to the most brilliant jade craftspeople in the world.
Finally, after sometimes years of intricate carving, the jade is sold in major retail centers like Hong Kong or Beijing. Once a finished product, the jade can sell for up to 10x its original price, creating wealth throughout the value chain.
As an example: the Polar Pride Boulder was carved into a massive Buddha and sold for $1 million in 2004.
Lithium
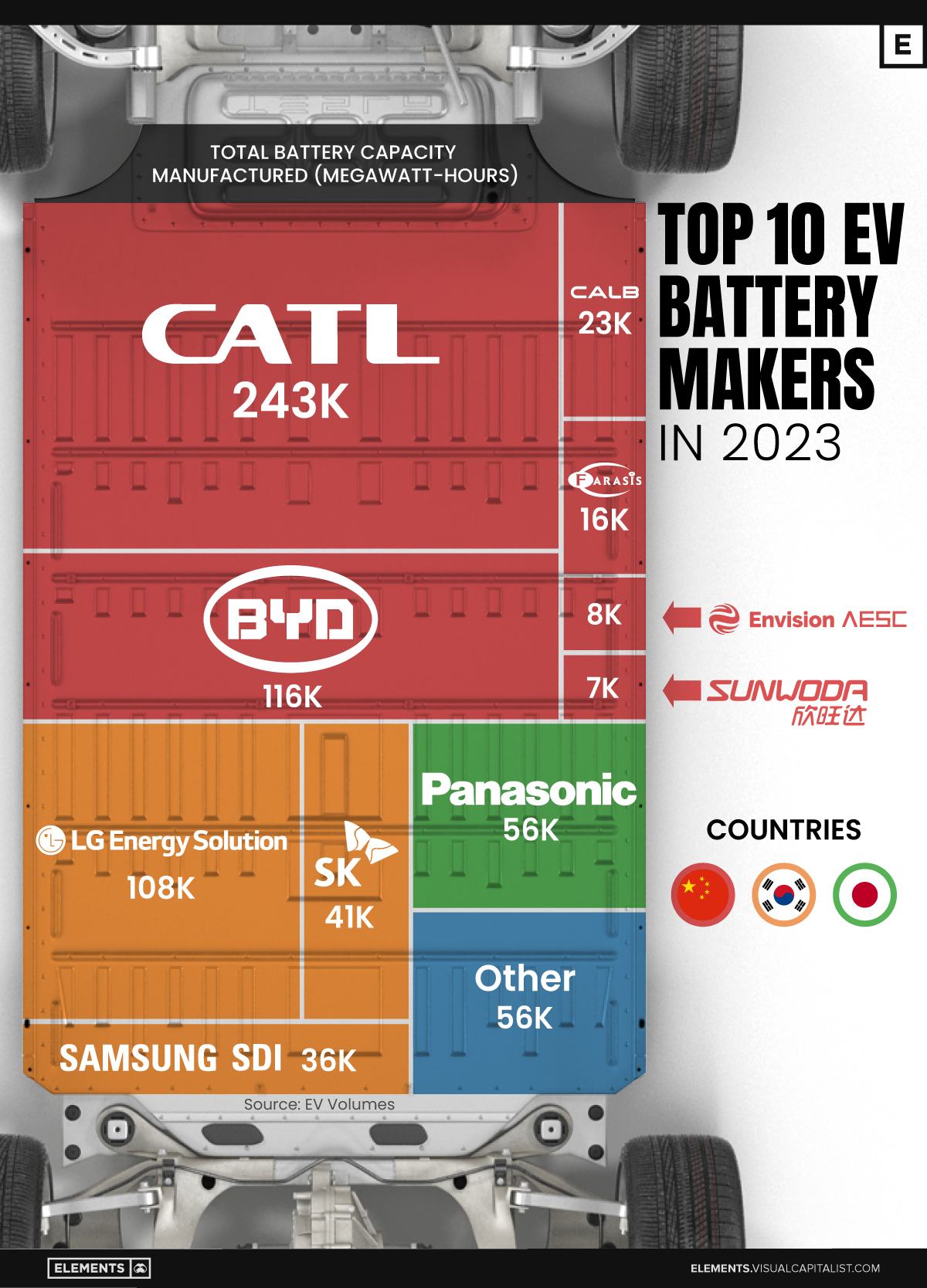
Ranked: The Top 10 EV Battery Manufacturers in 2023
Asia dominates this ranking of the world’s largest EV battery manufacturers in 2023.
The Top 10 EV Battery Manufacturers in 2023
This was originally posted on our Voronoi app. Download the app for free on iOS or Android and discover incredible data-driven charts from a variety of trusted sources.
Despite efforts from the U.S. and EU to secure local domestic supply, all major EV battery manufacturers remain based in Asia.
In this graphic we rank the top 10 EV battery manufacturers by total battery deployment (measured in megawatt-hours) in 2023. The data is from EV Volumes.
Chinese Dominance
Contemporary Amperex Technology Co. Limited (CATL) has swiftly risen in less than a decade to claim the title of the largest global battery group.
The Chinese company now has a 34% share of the market and supplies batteries to a range of made-in-China vehicles, including the Tesla Model Y, SAIC’s MG4/Mulan, and various Li Auto models.
| Company | Country | 2023 Production (megawatt-hour) | Share of Total Production |
|---|---|---|---|
| CATL | 🇨🇳 China | 242,700 | 34% |
| BYD | 🇨🇳 China | 115,917 | 16% |
| LG Energy Solution | 🇰🇷 Korea | 108,487 | 15% |
| Panasonic | 🇯🇵 Japan | 56,560 | 8% |
| SK On | 🇰🇷 Korea | 40,711 | 6% |
| Samsung SDI | 🇰🇷 Korea | 35,703 | 5% |
| CALB | 🇨🇳 China | 23,493 | 3% |
| Farasis Energy | 🇨🇳 China | 16,527 | 2% |
| Envision AESC | 🇨🇳 China | 8,342 | 1% |
| Sunwoda | 🇨🇳 China | 6,979 | 1% |
| Other | - | 56,040 | 8% |
In 2023, BYD surpassed LG Energy Solution to claim second place. This was driven by demand from its own models and growth in third-party deals, including providing batteries for the made-in-Germany Tesla Model Y, Toyota bZ3, Changan UNI-V, Venucia V-Online, as well as several Haval and FAW models.
The top three battery makers (CATL, BYD, LG) collectively account for two-thirds (66%) of total battery deployment.
Once a leader in the EV battery business, Panasonic now holds the fourth position with an 8% market share, down from 9% last year. With its main client, Tesla, now sourcing batteries from multiple suppliers, the Japanese battery maker seems to be losing its competitive edge in the industry.
Overall, the global EV battery market size is projected to grow from $49 billion in 2022 to $98 billion by 2029, according to Fortune Business Insights.
-
 Energy1 week ago
Energy1 week agoThe World’s Biggest Nuclear Energy Producers
-

 Money2 weeks ago
Money2 weeks agoWhich States Have the Highest Minimum Wage in America?
-
 Technology2 weeks ago
Technology2 weeks agoRanked: Semiconductor Companies by Industry Revenue Share
-
 Markets2 weeks ago
Markets2 weeks agoRanked: The World’s Top Flight Routes, by Revenue
-

 Countries2 weeks ago
Countries2 weeks agoPopulation Projections: The World’s 6 Largest Countries in 2075
-
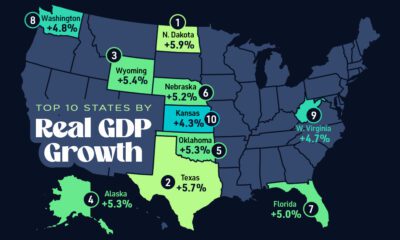
 Markets2 weeks ago
Markets2 weeks agoThe Top 10 States by Real GDP Growth in 2023
-
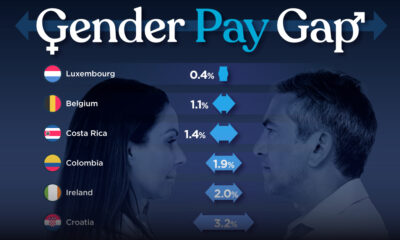
 Demographics2 weeks ago
Demographics2 weeks agoThe Smallest Gender Wage Gaps in OECD Countries
-
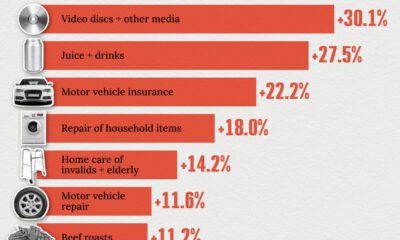
 United States2 weeks ago
United States2 weeks agoWhere U.S. Inflation Hit the Hardest in March 2024