Mining
More Than a Precious Metal: How Platinum Improves Our World
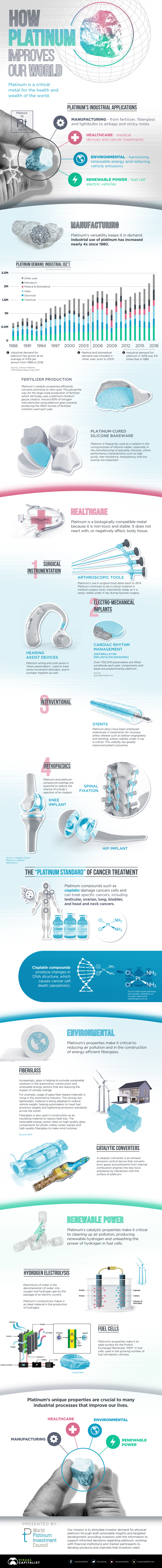
How Platinum Improves Our World
Within the hierarchy of precious metals, there is only one metal that could arguably stand above gold, and that is platinum.
This is due in large part to the metal’s rarity throughout history. Its earliest known use was on the Casket of Thebes in Ancient Egypt. South American Indigenous populations also used platinum for jewelry.
But platinum’s value goes beyond being a precious metal—its specific properties have made it indispensable to the modern economy, improving both the health and the wealth of the world.
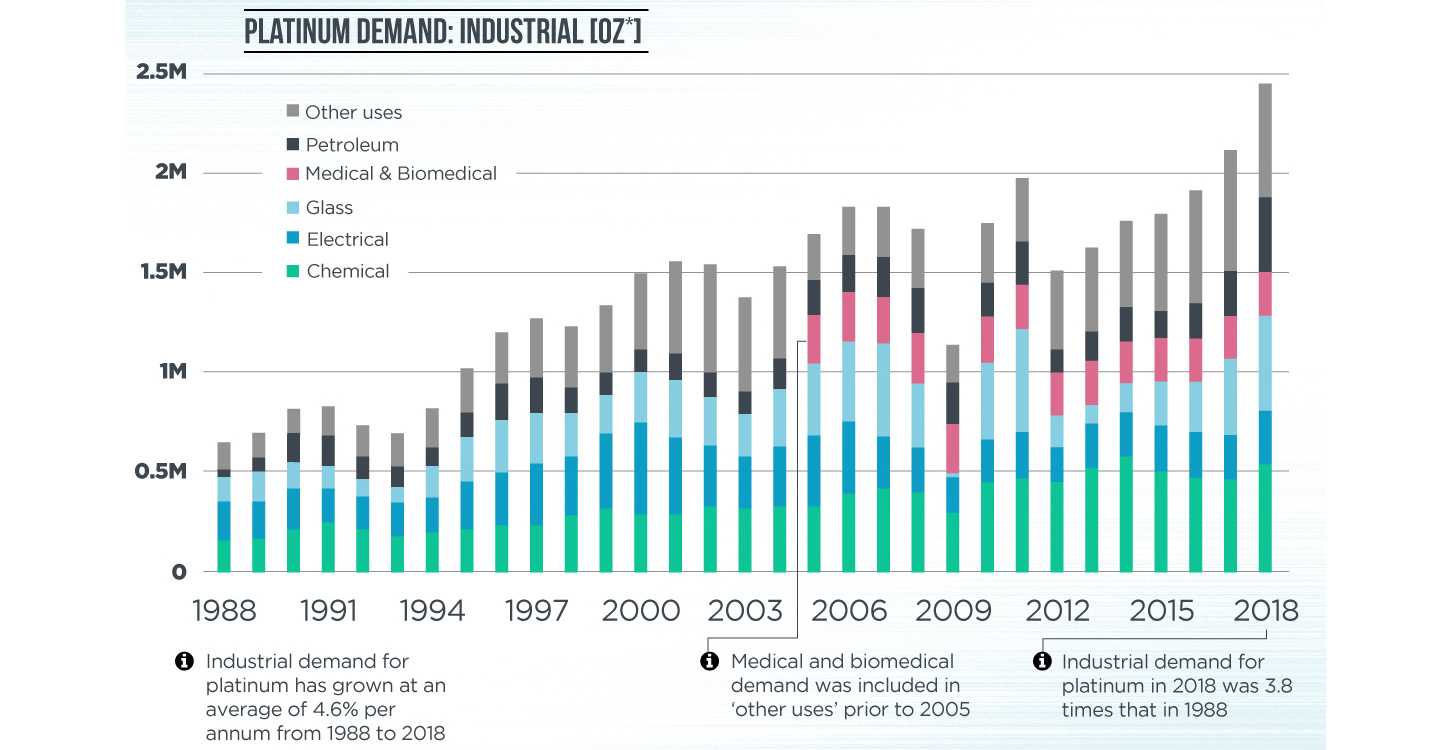
Platinum’s Industrial Applications
Today’s infographic comes to us from the World Platinum Investment Council and outlines specifically how specific platinum’s properties are used in the modern economy.
There are four primary uses of platinum aside from investment demand.
- Manufacturing
- Healthcare
- Environmental
- Renewable Power
Let’s look into all of these cases a little deeper.
1. Manufacturing
Platinum’s versatility in manufacturing has quadrupled its demand since 1980. Its catalytic properties are critical to the production of fertilizers, and more specifically, platinum’s efficiency in converting ammonia to nitric acid paved the way for large-scale fertilizer production.
Around 90% of the nitrogen produced using platinum catalysts is used to make 190 million tonnes of fertilizers each year.
2. Healthcare
Platinum is a biologically compatible metal because it is both non-toxic and stable. It does not react negatively with or affect body tissues, which makes it an ideal material for medical tools. Platinum’s use in medicine dates back to 1874 for its use in arthroscopic tools. Its stability also makes it ideal for pacemakers and hearing assist devices today.
While non-threatening to healthy cells, platinum compounds known as cisplatin can damage cancer cells and treat testicular, ovarian, lung, bladder, and other cancers. Given these crucial applications, the World Health Organization has put cisplatin on its List of Essential Medicines.
3. Environmental
Platinum is a critical material in the fight for cleaner air and in the construction of energy-efficient fiberglass. It is used in catalytic converters in exhaust systems of gas-powered vehicles, reducing the emission of harmful pollutants. In addition, platinum is used in the manufacturing process of high-end glass that improves the heating and cooling efficiency of homes and offices.
4. Renewable Power
Platinum’s catalytic properties make it critical to cleaning up air pollution, producing renewable hydrogen, and unleashing its power in fuel cells. Electrolysis, which can turn water into hydrogen and oxygen, works best when passing an electric current through platinum electrodes.
Fuel cells are set to power a new generation of emission-free vehicles, and platinum membranes are used inside of them as well.
More Than Precious
More than a precious metal, platinum has many applications that make it a critical material for the modern economy in years to come.
Lithium
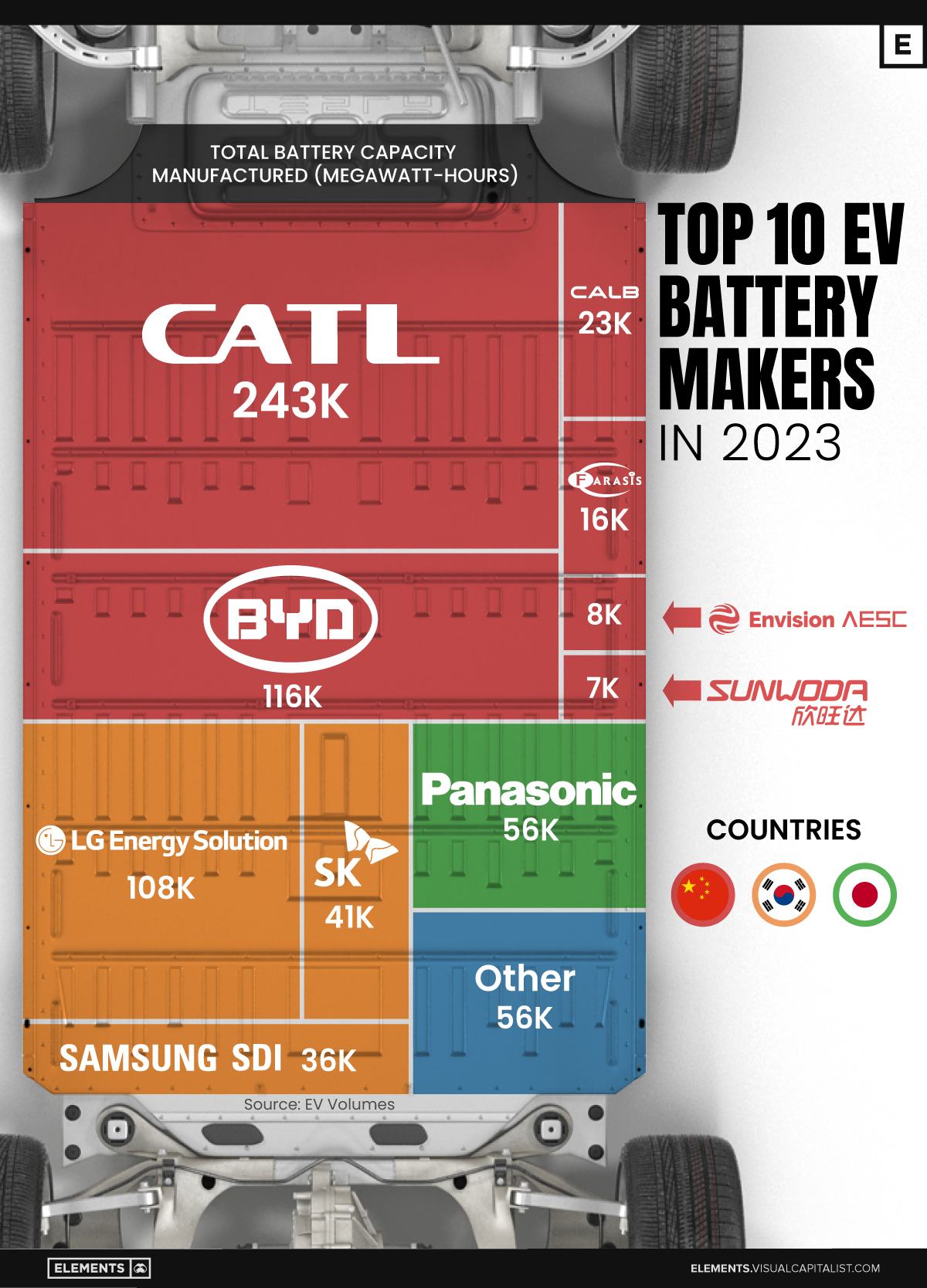
Ranked: The Top 10 EV Battery Manufacturers in 2023
Asia dominates this ranking of the world’s largest EV battery manufacturers in 2023.
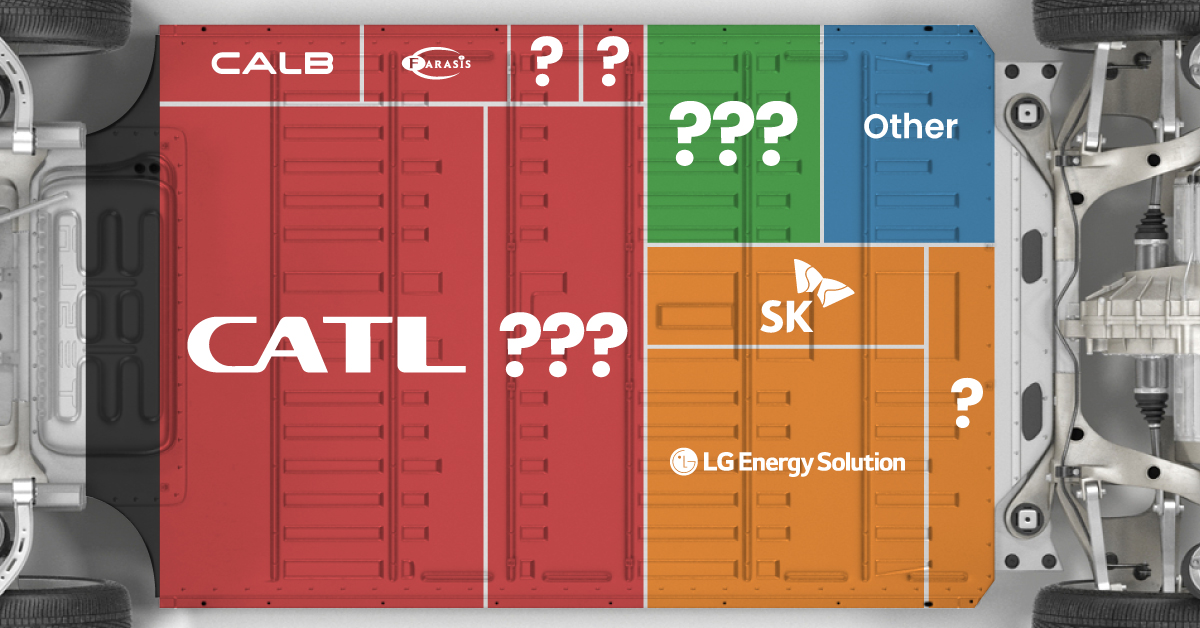
The Top 10 EV Battery Manufacturers in 2023
This was originally posted on our Voronoi app. Download the app for free on iOS or Android and discover incredible data-driven charts from a variety of trusted sources.
Despite efforts from the U.S. and EU to secure local domestic supply, all major EV battery manufacturers remain based in Asia.
In this graphic we rank the top 10 EV battery manufacturers by total battery deployment (measured in megawatt-hours) in 2023. The data is from EV Volumes.
Chinese Dominance
Contemporary Amperex Technology Co. Limited (CATL) has swiftly risen in less than a decade to claim the title of the largest global battery group.
The Chinese company now has a 34% share of the market and supplies batteries to a range of made-in-China vehicles, including the Tesla Model Y, SAIC’s MG4/Mulan, and various Li Auto models.
| Company | Country | 2023 Production (megawatt-hour) | Share of Total Production |
|---|---|---|---|
| CATL | 🇨🇳 China | 242,700 | 34% |
| BYD | 🇨🇳 China | 115,917 | 16% |
| LG Energy Solution | 🇰🇷 Korea | 108,487 | 15% |
| Panasonic | 🇯🇵 Japan | 56,560 | 8% |
| SK On | 🇰🇷 Korea | 40,711 | 6% |
| Samsung SDI | 🇰🇷 Korea | 35,703 | 5% |
| CALB | 🇨🇳 China | 23,493 | 3% |
| Farasis Energy | 🇨🇳 China | 16,527 | 2% |
| Envision AESC | 🇨🇳 China | 8,342 | 1% |
| Sunwoda | 🇨🇳 China | 6,979 | 1% |
| Other | - | 56,040 | 8% |
In 2023, BYD surpassed LG Energy Solution to claim second place. This was driven by demand from its own models and growth in third-party deals, including providing batteries for the made-in-Germany Tesla Model Y, Toyota bZ3, Changan UNI-V, Venucia V-Online, as well as several Haval and FAW models.
The top three battery makers (CATL, BYD, LG) collectively account for two-thirds (66%) of total battery deployment.
Once a leader in the EV battery business, Panasonic now holds the fourth position with an 8% market share, down from 9% last year. With its main client, Tesla, now sourcing batteries from multiple suppliers, the Japanese battery maker seems to be losing its competitive edge in the industry.
Overall, the global EV battery market size is projected to grow from $49 billion in 2022 to $98 billion by 2029, according to Fortune Business Insights.
-
 Debt1 week ago
Debt1 week agoHow Debt-to-GDP Ratios Have Changed Since 2000
-
 Markets2 weeks ago
Markets2 weeks agoRanked: The World’s Top Flight Routes, by Revenue
-

 Countries2 weeks ago
Countries2 weeks agoPopulation Projections: The World’s 6 Largest Countries in 2075
-
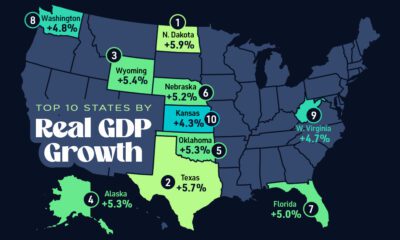
 Markets2 weeks ago
Markets2 weeks agoThe Top 10 States by Real GDP Growth in 2023
-
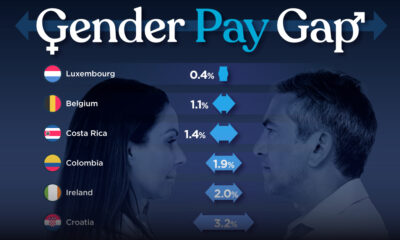
 Demographics2 weeks ago
Demographics2 weeks agoThe Smallest Gender Wage Gaps in OECD Countries
-
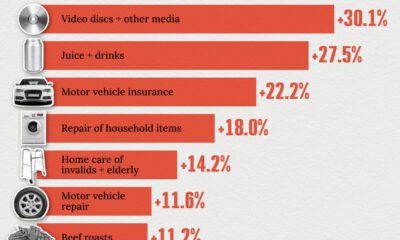
 United States2 weeks ago
United States2 weeks agoWhere U.S. Inflation Hit the Hardest in March 2024
-

 Green2 weeks ago
Green2 weeks agoTop Countries By Forest Growth Since 2001
-
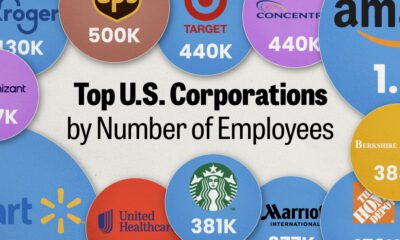
 United States2 weeks ago
United States2 weeks agoRanked: The Largest U.S. Corporations by Number of Employees