Mining
The Silver Series: World’s Growing Demand For Silver (Part 3 of 4)

Silver Series Part 3: The World’s Growing Demand For Silver
Silver is the most versatile metal in the world. Not only does it have the highest thermal and electrical conductivity of all metals, but it also has many other impressive properties: silver is antibacterial, durable, reflective, and malleable.
With such a multitude of significant material qualities, it is no surprise that now more than half of silver used today is in industrial processes. Last year, it is estimated that 53% of silver was used in industry – an increase from a total of 46% a decade ago.
Industry
Perhaps the most notable industrial sector for silver demand is photovoltaics, where 2.8 million oz of silver is used for every gigawatt of solar energy capacity. The total installed capacity of solar globally is at around 178 GW in 2014, and growth in global installs is also significant, gaining 14% between 2013 and 2014.
The metal’s other main industrial uses include brazing and soldering as well as fabrication. In the former category, using silver for brazing and soldering helps produce leak-tight and corrosion-resistant joints when combining metal parts.
In terms of fabrication, silver-containing vehicles, batteries, and chemical processes are the most important categories for future growth. For use in automotive manufacturing, which has the highest project growth (4.9% CAGR) of categories other than solar, silver is used to coat electrical contacts to ensure the most efficient energy flow. Silver batteries, which have similar energy densities to lithium-ion batteries, are used in military and aerospace applications because they are more reliable and safe. Lastly, silver catalysts are also used to help combine ethylene and oxygen together to create ethylene oxide, which is used in medicine, anti-freeze, and cosmetics.
Investment
While industrial uses are the most prominent for the metal, it is investment that has been the real growth engine for silver demand over the last decade.
In 2014, 20% of all silver is used for investment purposes, compared to only 7% a decade ago. The demand for silver coins and bars has more than quadrupled since the early 2000s, and the coin sales of Canadian Maple Leafs and American Eagles have been soaring for years.
It is also interesting to note, especially at a time of such market vulnerability, that the ratio of silver to gold ounces bought in the market increases. This ratio peaked recently during the Global Financial Crisis in 2008, and in the last 12 months it has jumped up to comparable levels.
Jewelry
Jewelry is also a crucial market for silver, and the category is considered by some to serve as an investment and store of wealth as well. Lower prices for silver in recent years have helped jewelry rebound in Asia and the United States in particular.
Globally, silver jewelry fabrication experienced its second year of consecutive growth, increasing 1.5% to achieve a new record high. This was a reflection chiefly of the strong performance of silver jewelry demand from India, which surged 47% from 2013 levels.
A record of 7,063 tonnes of silver were imported to India in 2014, up 15% from 2013. The country imported more silver in November 2014 than they did in all of 2009. This is partially due to India’s rising population and per capital income, and also due to import restrictions on gold in the world’s second most populous country.
Conclusion
Silver demand is multi-faceted, with just over half of demand coming from industry and the rest split between mainly investment and jewelry demand. We will cover the historical returns of investing in silver in-depth with our final part of the Silver Series in the coming weeks.
Don’t miss out on the last part of the Silver Series by connecting with Visual Capitalist.
Lithium
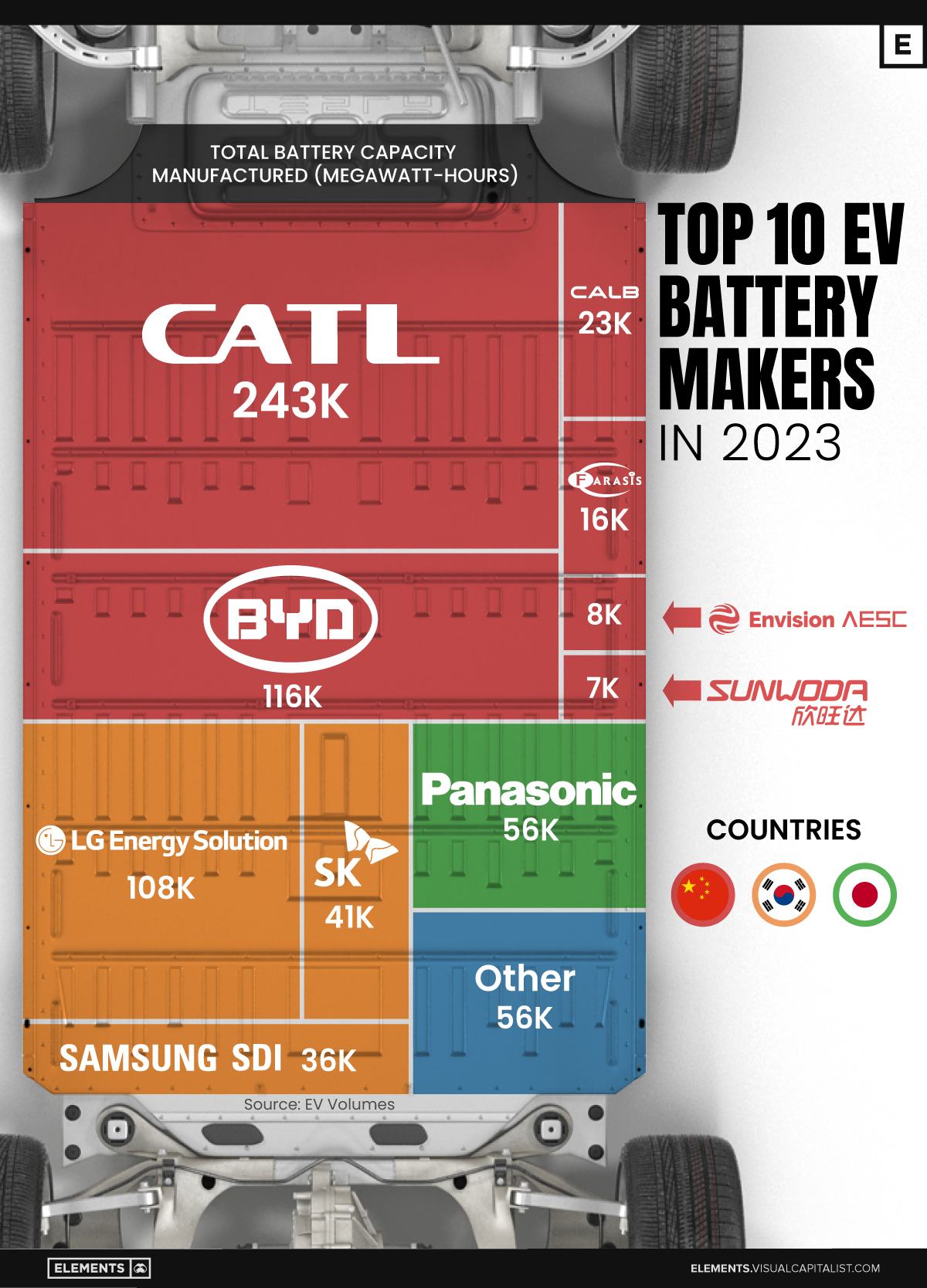
Ranked: The Top 10 EV Battery Manufacturers in 2023
Asia dominates this ranking of the world’s largest EV battery manufacturers in 2023.
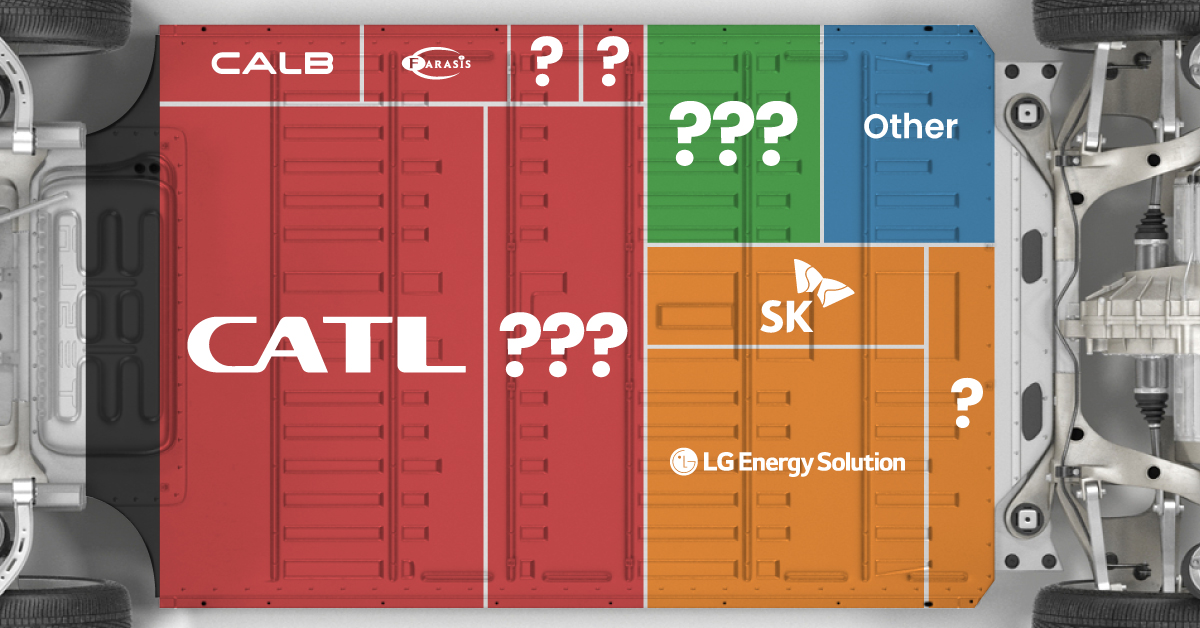
The Top 10 EV Battery Manufacturers in 2023
This was originally posted on our Voronoi app. Download the app for free on iOS or Android and discover incredible data-driven charts from a variety of trusted sources.
Despite efforts from the U.S. and EU to secure local domestic supply, all major EV battery manufacturers remain based in Asia.
In this graphic we rank the top 10 EV battery manufacturers by total battery deployment (measured in megawatt-hours) in 2023. The data is from EV Volumes.
Chinese Dominance
Contemporary Amperex Technology Co. Limited (CATL) has swiftly risen in less than a decade to claim the title of the largest global battery group.
The Chinese company now has a 34% share of the market and supplies batteries to a range of made-in-China vehicles, including the Tesla Model Y, SAIC’s MG4/Mulan, and various Li Auto models.
| Company | Country | 2023 Production (megawatt-hour) | Share of Total Production |
|---|---|---|---|
| CATL | 🇨🇳 China | 242,700 | 34% |
| BYD | 🇨🇳 China | 115,917 | 16% |
| LG Energy Solution | 🇰🇷 Korea | 108,487 | 15% |
| Panasonic | 🇯🇵 Japan | 56,560 | 8% |
| SK On | 🇰🇷 Korea | 40,711 | 6% |
| Samsung SDI | 🇰🇷 Korea | 35,703 | 5% |
| CALB | 🇨🇳 China | 23,493 | 3% |
| Farasis Energy | 🇨🇳 China | 16,527 | 2% |
| Envision AESC | 🇨🇳 China | 8,342 | 1% |
| Sunwoda | 🇨🇳 China | 6,979 | 1% |
| Other | - | 56,040 | 8% |
In 2023, BYD surpassed LG Energy Solution to claim second place. This was driven by demand from its own models and growth in third-party deals, including providing batteries for the made-in-Germany Tesla Model Y, Toyota bZ3, Changan UNI-V, Venucia V-Online, as well as several Haval and FAW models.
The top three battery makers (CATL, BYD, LG) collectively account for two-thirds (66%) of total battery deployment.
Once a leader in the EV battery business, Panasonic now holds the fourth position with an 8% market share, down from 9% last year. With its main client, Tesla, now sourcing batteries from multiple suppliers, the Japanese battery maker seems to be losing its competitive edge in the industry.
Overall, the global EV battery market size is projected to grow from $49 billion in 2022 to $98 billion by 2029, according to Fortune Business Insights.
-
 Debt1 week ago
Debt1 week agoHow Debt-to-GDP Ratios Have Changed Since 2000
-
 Markets2 weeks ago
Markets2 weeks agoRanked: The World’s Top Flight Routes, by Revenue
-

 Countries2 weeks ago
Countries2 weeks agoPopulation Projections: The World’s 6 Largest Countries in 2075
-
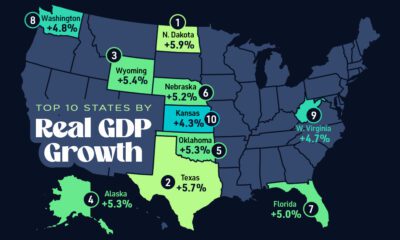
 Markets2 weeks ago
Markets2 weeks agoThe Top 10 States by Real GDP Growth in 2023
-
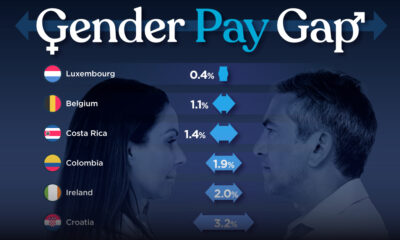
 Demographics2 weeks ago
Demographics2 weeks agoThe Smallest Gender Wage Gaps in OECD Countries
-
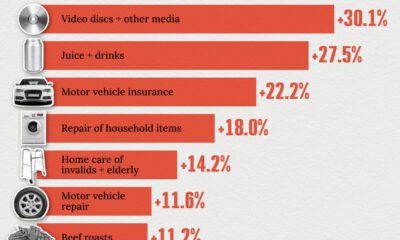
 Economy2 weeks ago
Economy2 weeks agoWhere U.S. Inflation Hit the Hardest in March 2024
-

 Green2 weeks ago
Green2 weeks agoTop Countries By Forest Growth Since 2001
-
 United States2 weeks ago
United States2 weeks agoRanked: The Largest U.S. Corporations by Number of Employees