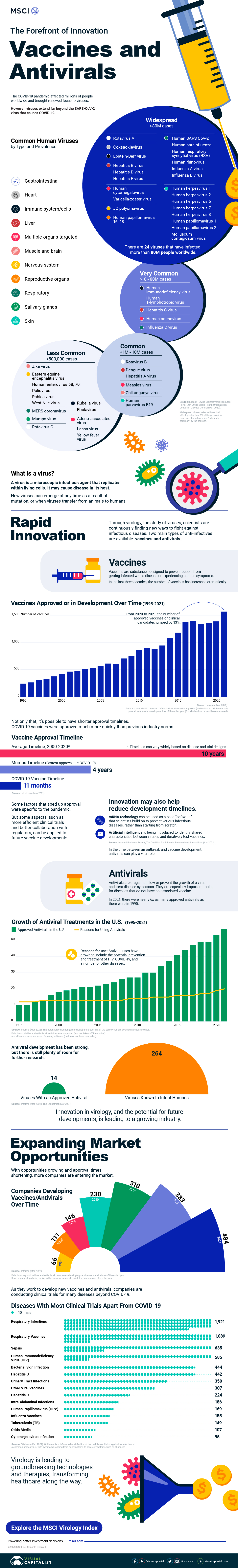
Innovation in Virology: Vaccines and Antivirals
Innovation in Virology: Vaccines and Antivirals
The COVID-19 pandemic affected millions of people worldwide and brought renewed focus to virology—the study of viruses.
However, impact made by viruses extends far beyond the SARS-CoV-2 virus that causes COVID-19. There are 24 viruses that have each infected more than 80 million people globally, from hepatitis to influenza.
In this graphic from MSCI, we uncover innovation in vaccines and antivirals and the related market opportunities.
What is a Virus?
A virus is a microscopic infectious agent that replicates within living cells. It may cause disease in its host. New viruses can emerge at any time as a result of mutation, or when viruses transfer from animals to humans.
Through virology, scientists are continuously finding new ways to fight against infectious diseases. Two main types of anti-infectives are available: vaccines and antivirals.
Rapid Innovation in Vaccines
Vaccines are substances designed to prevent people from getting infected with a disease or experiencing serious symptoms.
The number of vaccines has increased dramatically over the last three decades. From 2020 to 2021 alone, the number of approved vaccines or clinical candidates jumped by 13%.
| Year | Vaccines Approved or in Development |
|---|---|
| 1995 | 240 |
| 1996 | 262 |
| 1997 | 309 |
| 1998 | 323 |
| 1999 | 374 |
| 2000 | 415 |
| 2001 | 462 |
| 2002 | 472 |
| 2003 | 509 |
| 2004 | 531 |
| 2005 | 564 |
| 2006 | 610 |
| 2007 | 606 |
| 2008 | 704 |
| 2009 | 751 |
| 2010 | 866 |
| 2011 | 893 |
| 2012 | 880 |
| 2013 | 943 |
| 2014 | 1075 |
| 2015 | 1179 |
| 2016 | 1374 |
| 2017 | 1397 |
| 2018 | 1340 |
| 2019 | 1356 |
| 2020 | 1388 |
| 2021 | 1567 |
Data is a snapshot in time and reflects all vaccines ever approved (and not taken off the market) plus all vaccines in development as of the noted year (for which a trial has not been canceled).
Not only that, it’s possible to have shorter approval timelines. COVID-19 vaccines were approved within 11 months, much more quickly than the 2000-2020 average of 10 years.
In the time between an outbreak and vaccine development, antivirals can play a vital role.
Antivirals: The Second Line of Defense in Virology
Antivirals are drugs that slow or prevent the growth of a virus and treat disease symptoms. They are especially important tools for diseases that do not have an associated vaccine.
In 2021, there were nearly six times as many approved antivirals as there were in 1995. Not only that, antiviral uses have grown to include the potential prevention and treatment of HIV, COVID-19, and a number of other diseases.
| Year | Approved Antivirals in the U.S. | Reasons for Using Antivirals |
|---|---|---|
| 1995 | 10 | 12 |
| 1996 | 10 | 12 |
| 1997 | 12 | 12 |
| 1998 | 13 | 13 |
| 1999 | 16 | 13 |
| 2000 | 18 | 13 |
| 2001 | 19 | 13 |
| 2002 | 20 | 13 |
| 2003 | 21 | 13 |
| 2004 | 21 | 13 |
| 2005 | 22 | 13 |
| 2006 | 23 | 13 |
| 2007 | 24 | 13 |
| 2008 | 26 | 13 |
| 2009 | 27 | 14 |
| 2010 | 27 | 14 |
| 2011 | 30 | 14 |
| 2012 | 30 | 15 |
| 2013 | 34 | 15 |
| 2014 | 37 | 15 |
| 2015 | 41 | 16 |
| 2016 | 44 | 16 |
| 2017 | 47 | 16 |
| 2018 | 49 | 17 |
| 2019 | 49 | 17 |
| 2020 | 53 | 19 |
| 2021 | 57 | 20 |
The potential prevention (prophylaxis) and treatment of the same virus are counted as separate uses. Data is cumulative and reflects all antivirals ever approved (and not taken off the market) and all reasons ever approved for using antivirals (that have not been rescinded).
Innovation in virology—and the potential for future developments—is leading to a growing industry.
Expanding Market Opportunities
With opportunities growing and approval times shortening, more companies are entering the market.
| Year | Companies Developing Vaccines/Antivirals |
|---|---|
| 1995 | 66 |
| 1996 | 73 |
| 1997 | 80 |
| 1998 | 81 |
| 1999 | 87 |
| 2000 | 111 |
| 2001 | 125 |
| 2002 | 140 |
| 2003 | 154 |
| 2004 | 144 |
| 2005 | 146 |
| 2006 | 163 |
| 2007 | 167 |
| 2008 | 196 |
| 2009 | 203 |
| 2010 | 230 |
| 2011 | 237 |
| 2012 | 255 |
| 2013 | 277 |
| 2014 | 289 |
| 2015 | 310 |
| 2016 | 362 |
| 2017 | 392 |
| 2018 | 374 |
| 2019 | 370 |
| 2020 | 383 |
| 2021 | 484 |
Data is a snapshot in time and reflects all companies developing vaccines or antivirals as of the noted year. If a company stops being active in the space or ceases to exist, they are removed from the total.
As they work to develop new vaccines and antivirals, companies are conducting clinical trials for many diseases beyond COVID-19 such as respiratory infections and sepsis.
Virology is leading to a number of groundbreaking technologies and therapies, transforming healthcare along the way.

Explore the MSCI Virology Index now.

-

 Healthcare1 month ago
Healthcare1 month agoThe Cost of an EpiPen in Major Markets
This visualization compares EpiPen prices around the world, with the U.S. having the highest prices by far.
-

 Healthcare3 months ago
Healthcare3 months agoCharted: Global Tobacco Use by Country and Sex
This visual shows tobacco use by country and sex, highlighting which countries still have a high prevalence of smoking.
-
 Healthcare4 months ago
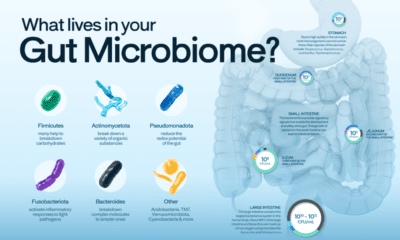
Healthcare4 months agoVisualized: What Lives in Your Gut Microbiome?
The human gut microbiome contains a world of microbes. We look at the the bacteria that deeply affect our health and well-being.
-
 Healthcare5 months ago
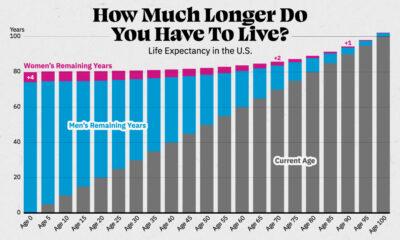
Healthcare5 months agoCharted: Average Years Left to Live by Age
Visualizing the number of years left to live for Americans at every age, reveals the broader trends in American life expectancy.
-

 Healthcare5 months ago
Healthcare5 months agoCharted: The Average Cost of Insulin By Country
This visual highlights the cost of insulin by country, showing how much more expensive diabetes medicine is in the U.S.
-
 Personal Finance12 months ago
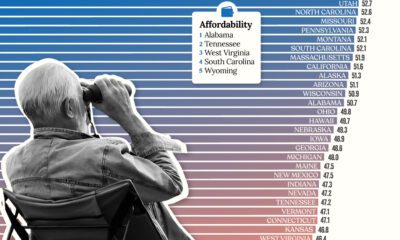
Personal Finance12 months agoRanked: The Best U.S. States for Retirement
Getting ready for retirement? See which states score the highest in terms of affordability, quality of life, and health care.