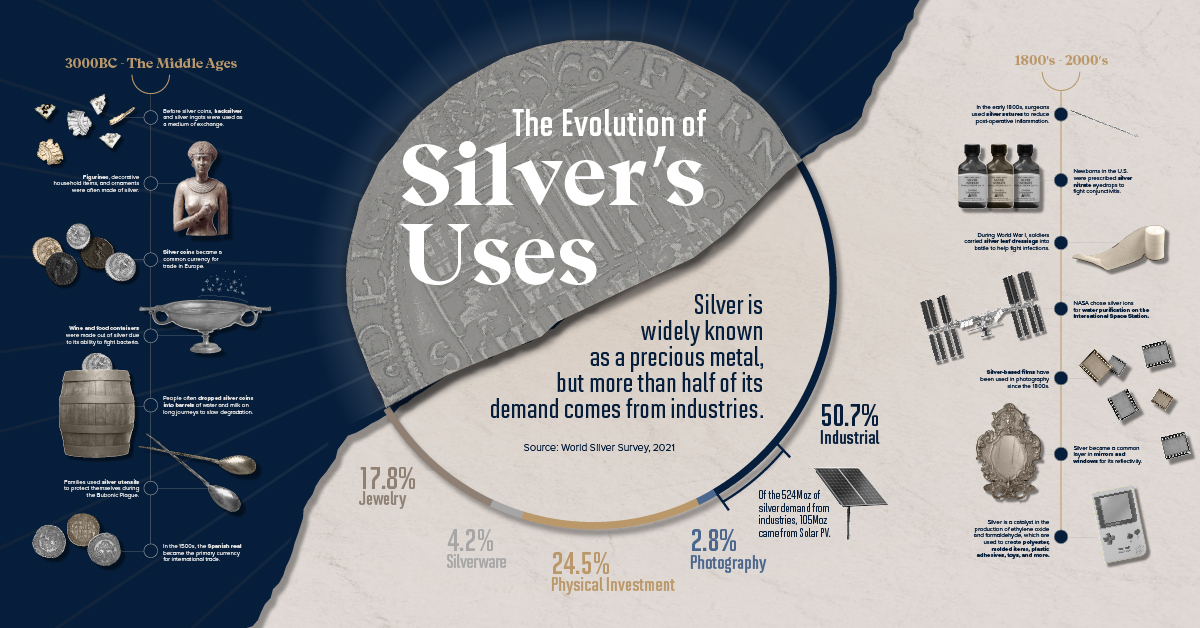
Silver Through the Ages: The Uses of Silver Over Time
The following content is sponsored by Blackrock Silver.
Silver is one of the most versatile metals on Earth, with a unique combination of uses both as a precious and industrial metal.
Today, silver’s uses span many modern technologies, including solar panels, electric vehicles, and 5G devices. However, the uses of silver in currency, medicine, art, and jewelry have helped advance civilization, trade, and technology for thousands of years.
The Uses of Silver Over Time
The below infographic from Blackrock Silver takes us on a journey of silver’s uses through time, from the past to the future.

3,000 BC – The Middle Ages
The earliest accounts of silver can be traced to 3,000 BC in modern-day Turkey, where its mining spurred trade in the ancient Aegean and Mediterranean seas. Traders and merchants would use hacksilver—rough-cut pieces of silver—as a medium of exchange for goods and services.
Around 1,200 BC, the Ancient Greeks began refining and minting silver coins from the rich deposits found in the mines of Laurion just outside Athens. By 100 BC, modern-day Spain became the center of silver mining for the Roman Empire while silver bullion traveled along the Asian spice trade routes. By the late 1400s, Spain brought its affinity for silver to the New World where it uncovered the largest deposits of silver in history in the dusty hills of Bolivia.
Besides the uses of silver in commerce, people also recognized silver’s ability to fight bacteria. For instance, wine and food containers were often made out of silver to prevent spoilage. In addition, during breakouts of the Bubonic plague in medieval and renaissance Europe, people ate and drank with silver utensils to protect themselves from disease.
The 1800s – 2000s
New medicinal uses of silver came to light in the 19th and 20th centuries. Surgeons stitched post-operative wounds with silver sutures to reduce inflammation. In the early 1900s, doctors prescribed silver nitrate eyedrops to prevent conjunctivitis in newborn babies. Furthermore, in the 1960s, NASA developed a water purifier that dispensed silver ions to kill bacteria and purify water on its spacecraft.
The Industrial Revolution drove the onset of silver’s industrial applications. Thanks to its high light sensitivity and reflectivity, it became a key ingredient in photographic films, windows, and mirrors. Even today, skyscraper windows are often coated with silver to reflect sunlight and keep interior spaces cool.
The 2000s – Present
The uses of silver have come a long way since hacksilver and utensils, evolving with time and technology.
Silver is the most electrically conductive metal, making it a natural choice for electronic devices. Almost every electronic device with a switch or button contains silver, from smartphones to electric vehicles. Solar panels also utilize silver as a conductive layer in photovoltaic cells to transport and store electricity efficiently.
In addition, it has several medicinal applications that range from treating burn wounds and ulcers to eliminating bacteria in air conditioning systems and clothes.
Silver for the Future
Silver has always been useful to industries and technologies due to its unique properties, from its antibacterial nature to high electrical conductivity. Today, silver is critical for the next generation of renewable energy technologies.
For every age, silver proves its value.
-
 Lithium4 days ago
Lithium4 days agoRanked: The Top 10 EV Battery Manufacturers in 2023
Asia dominates this ranking of the world's largest EV battery manufacturers in 2023.
-
 Mining1 week ago
Mining1 week agoGold vs. S&P 500: Which Has Grown More Over Five Years?
The price of gold has set record highs in 2024, but how has this precious metal performed relative to the S&P 500?
-
 Mining3 weeks ago
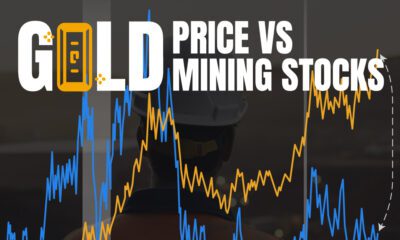
Mining3 weeks agoCharted: The Value Gap Between the Gold Price and Gold Miners
While the price of gold has reached new record highs in 2024, gold mining stocks are still far from their 2011 peaks.
-
 Uranium2 months ago
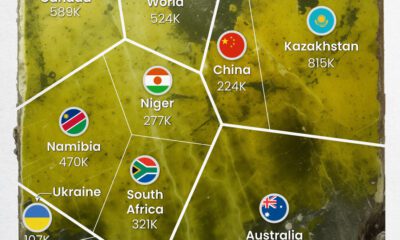
Uranium2 months agoCharted: Global Uranium Reserves, by Country
We visualize the distribution of the world's uranium reserves by country, with 3 countries accounting for more than half of total reserves.
-
 Energy4 months ago
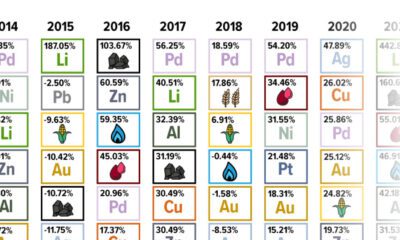
Energy4 months agoThe Periodic Table of Commodity Returns (2014-2023)
Commodity returns in 2023 took a hit. This graphic shows the performance of commodities like gold, oil, nickel, and corn over the last decade.
-
 Mining4 months ago
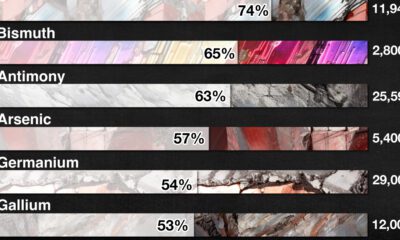
Mining4 months agoChina Dominates the Supply of U.S. Critical Minerals List
The U.S. Geological Survey estimates that in 2022, China was the world’s leading producer of 30 out of 50 entries on the U.S. critical minerals list.