Energy
Uranium: The Metal of Tomorrow
Nuclear power accounts for 5.7% of the world’s energy and 13% of the world’s electricity. Uranium, used in nuclear power, is a relatively clean source of energy that does not produce greenhouse emissions.
Uranium is extremely dense – it is nearly as heavy as gold. It is, however, about 500 times more common than gold in the earth’s crust.
This infographic covers the history of uranium, its properties, the supply and demand forecasts, the advantages and disadvantages of nuclear power, uranium as an energy source, and military applications.
Energy
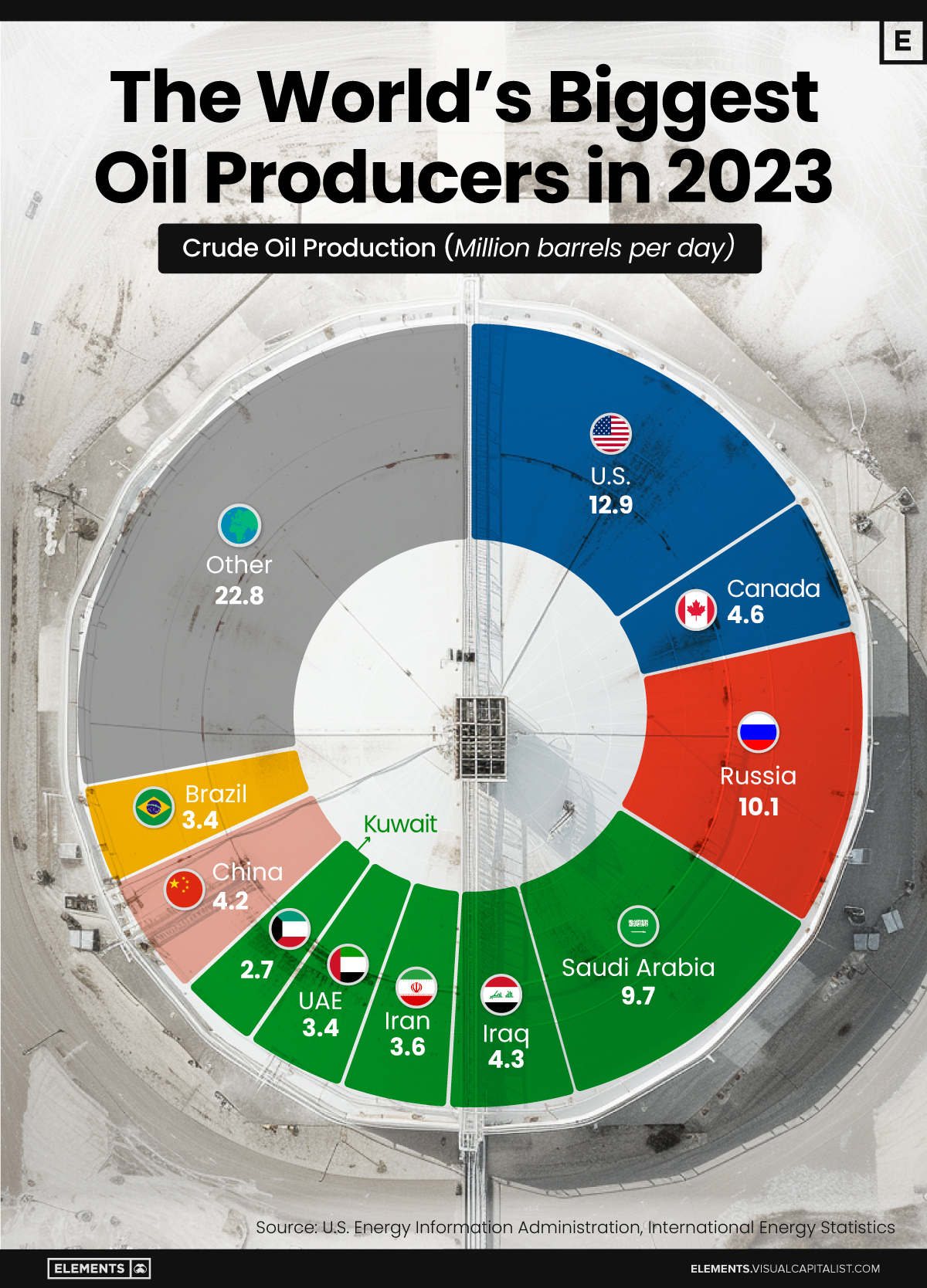
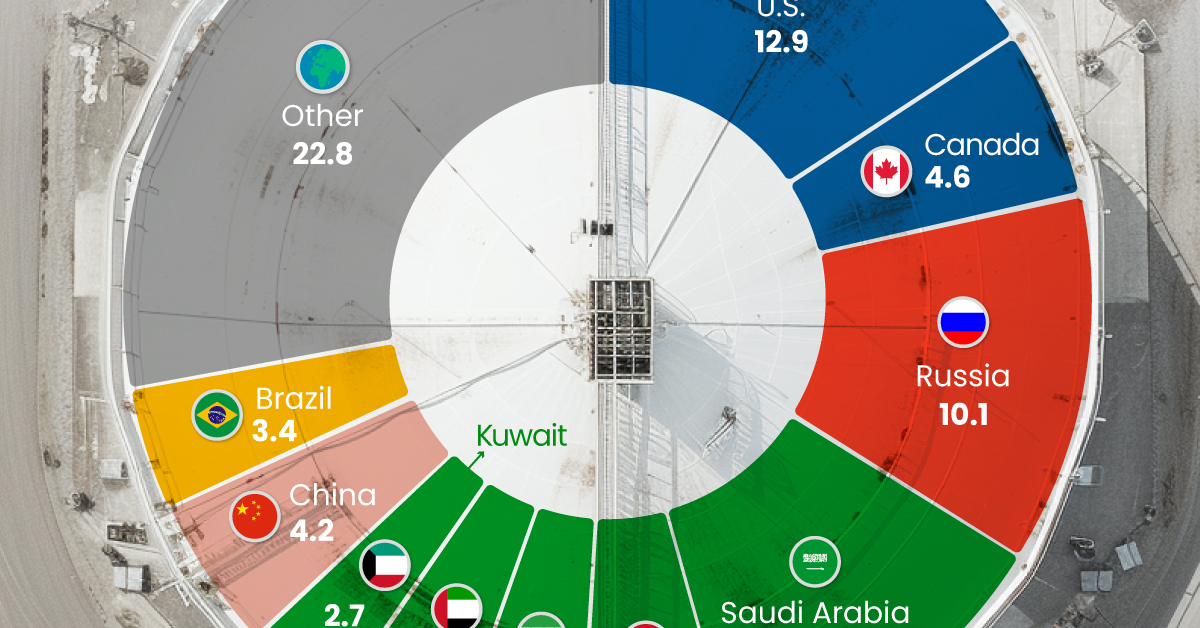
The World’s Biggest Oil Producers in 2023
Just three countries accounted for 40% of global oil production last year.
The World’s Biggest Oil Producers in 2023
This was originally posted on Elements. Sign up to the free mailing list to get beautiful visualizations on natural resource megatrends in your email.
Despite efforts to decarbonize the global economy, oil still remains one of the world’s most important resources. It’s also produced by a fairly limited group of countries, which can be a source of economic and political leverage.
This graphic illustrates global crude oil production in 2023, measured in million barrels per day, sourced from the U.S. Energy Information Administration (EIA).
Three Countries Account for 40% of Global Oil Production
In 2023, the United States, Russia, and Saudi Arabia collectively contributed 32.7 million barrels per day to global oil production.
| Oil Production 2023 | Million barrels per day |
|---|---|
| 🇺🇸 U.S. | 12.9 |
| 🇷🇺 Russia | 10.1 |
| 🇸🇦 Saudi Arabia | 9.7 |
| 🇨🇦 Canada | 4.6 |
| 🇮🇶 Iraq | 4.3 |
| 🇨🇳 China | 4.2 |
| 🇮🇷 Iran | 3.6 |
| 🇧🇷 Brazil | 3.4 |
| 🇦🇪 UAE | 3.4 |
| 🇰🇼 Kuwait | 2.7 |
| 🌍 Other | 22.8 |
These three nations have consistently dominated oil production since 1971. The leading position, however, has alternated among them over the past five decades.
In contrast, the combined production of the next three largest producers—Canada, Iraq, and China—reached 13.1 million barrels per day in 2023, just surpassing the production of the United States alone.
In the near term, no country is likely to surpass the record production achieved by the U.S. in 2023, as no other producer has ever reached a daily capacity of 13.0 million barrels. Recently, Saudi Arabia’s state-owned Saudi Aramco scrapped plans to increase production capacity to 13.0 million barrels per day by 2027.
In 2024, analysts forecast that the U.S. will maintain its position as the top oil producer. In fact, according to Macquarie Group, U.S. oil production is expected to achieve a record pace of about 14 million barrels per day by the end of the year.
-

 Technology2 weeks ago
Technology2 weeks agoRanked: The Most Popular Smartphone Brands in the U.S.
-
 Automotive1 week ago
Automotive1 week agoAlmost Every EV Stock is Down After Q1 2024
-

 Money1 week ago
Money1 week agoWhere Does One U.S. Tax Dollar Go?
-
 Green2 weeks ago
Green2 weeks agoRanked: Top Countries by Total Forest Loss Since 2001
-
 Real Estate2 weeks ago
Real Estate2 weeks agoVisualizing America’s Shortage of Affordable Homes
-
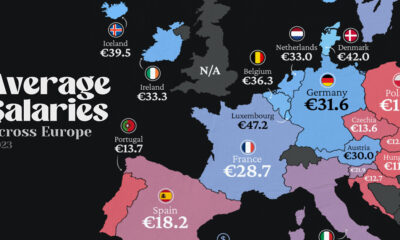
 Maps2 weeks ago
Maps2 weeks agoMapped: Average Wages Across Europe
-
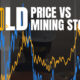 Mining2 weeks ago
Mining2 weeks agoCharted: The Value Gap Between the Gold Price and Gold Miners
-
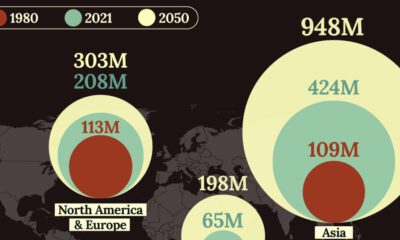
 Demographics2 weeks ago
Demographics2 weeks agoVisualizing the Size of the Global Senior Population