Titanium: What You Should Know and Why You Should Care
Titanium: What You Should Know and Why You Should Care
Titanium is a special metal that often flies under the radar.
Stronger than aluminum and lighter than steel, titanium has unique properties that have enabled its uses in a range of different industries like defense and medicine.
This infographic from our sponsor IperionX highlights what you need to know about titanium, from its unique properties to the metal’s uses in the modern economy. This is part one of three infographics in the Titanium 101 Series.
The Properties of Titanium
What makes titanium so special? Here are some of the properties that make titanium naturally superior to more common substitutes like stainless steel and aluminum:
- High strength-to-weight ratio:
Titanium is two times stronger than aluminum and 45% lighter than steel with comparable strength. - Resistance to corrosion:
Titanium’s natural resistance to corrosion allows for applications in harsh environments, including under seawater.
- Abundance:
Titanium is the 9th most abundant element in the Earth’s crust, found in nearly all rocks and sediments.
- Biocompatibility:
Titanium’s inertness inside the human body makes it biocompatible and suitable for medical and dental implants in humans.
- Temperature resistance:
Titanium has a melting point of 1,670 °C and can withstand temperatures higher and lower than stainless steel and aluminum.
Several industries harness titanium’s unique properties in different ways. While some rely on it for its natural resistance to corrosion, others make use of its high strength and low weight.
The Uses of Titanium
After titanium is mined and refined from the ground, it is primarily consumed in two forms. The majority of it, about 95% in the United States, is consumed in the form of titanium dioxide (TiO2). TiO2 is a brilliant white pigment used in everything from paints to food colorants and cosmetic products.
The remaining 5% of titanium is consumed in its metal form with highly specialized uses that also make it critical mineral for national security:
- Aerospace:
Titanium’s high strength and relatively lower weight make it suitable for airplane body frames (airframes) in combination with aluminum. It’s also used in turbofan engines for its temperature resistance. - Defense:
Naval ships and submarines make use of titanium’s unmatched corrosion resistance underwater, and ground vehicles like tanks are often partly plated with titanium parts. Additionally, many of the U.S. military’s aircraft are made with significant amounts of titanium alloys.
- Medical implants and prosthetics:
Titanium’s biocompatibility makes it especially useful for prosthetic parts like artificial joints, spinal fixtures, and dental implants.
- Seawater desalination plants:
Titanium is used in piping, tubing, and valves in seawater desalination plants due to its natural corrosion resistance.
- Clean energy:
Lithium-titanate batteries, found in some EVs, use titanium in the anode to allow for faster recharging. Additionally, titanium is also used in geothermal power plants to cope with the high heat and pressure from power generation.
The Titanium Age
Despite its superior properties and natural edge over other metals, titanium isn’t as widespread as stainless steel and aluminum, largely due to its high costs of production.
Titanium is currently difficult and expensive to produce because it is refined using the 80-year-old Kroll process, which is both energy- and carbon-intensive.
With a lower economic and environmental cost of production, titanium’s applications could spread far beyond its current high-performance uses, potentially allowing it to challenge the massive markets for stainless steel and aluminum.
In the next part of the Titanium 101 Series sponsored by IperionX, we will further explore titanium’s potential for growth and mainstream application in the future.

-
 Mining3 days ago
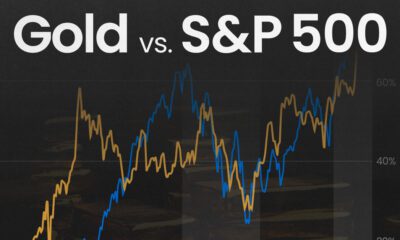
Mining3 days agoGold vs. S&P 500: Which Has Grown More Over Five Years?
The price of gold has set record highs in 2024, but how has this precious metal performed relative to the S&P 500?
-
 Mining2 weeks ago
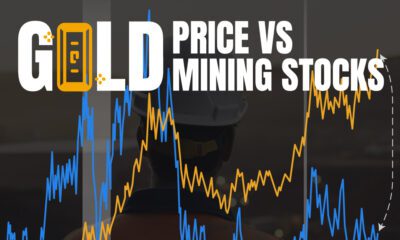
Mining2 weeks agoCharted: The Value Gap Between the Gold Price and Gold Miners
While the price of gold has reached new record highs in 2024, gold mining stocks are still far from their 2011 peaks.
-
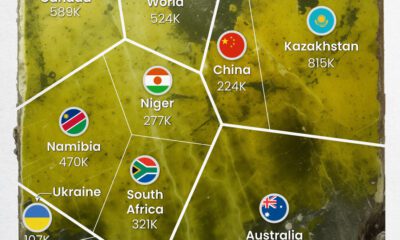
 Uranium2 months ago
Uranium2 months agoCharted: Global Uranium Reserves, by Country
We visualize the distribution of the world’s uranium reserves by country, with 3 countries accounting for more than half of total reserves.
-
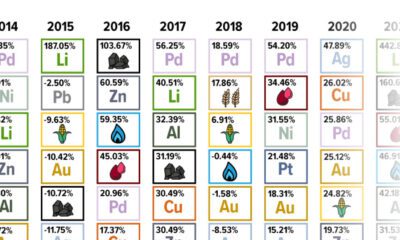
 Markets3 months ago
Markets3 months agoThe Periodic Table of Commodity Returns (2014-2023)
Commodity returns in 2023 took a hit. This graphic shows the performance of commodities like gold, oil, nickel, and corn over the last decade.
-
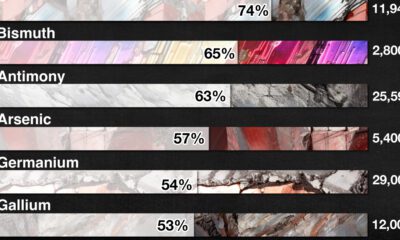
 Strategic Metals3 months ago
Strategic Metals3 months agoChina Dominates the Supply of U.S. Critical Minerals List
The U.S. Geological Survey estimates that in 2022, China was the world’s leading producer of 30 out of 50 entries on the U.S. critical minerals list.
-
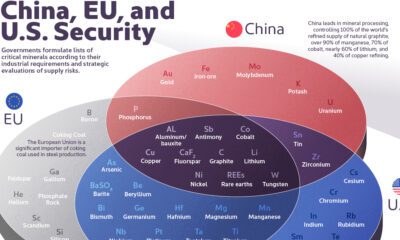
 Mining5 months ago
Mining5 months agoThe Critical Minerals to China, EU, and U.S. National Security
Ten materials, including cobalt, lithium, graphite, and rare earths, are deemed critical by all three.





