Lithium-Cobalt Batteries: Powering the Electric Vehicle Revolution
The following content is sponsored by Fuse Cobalt.
Lithium-Cobalt Batteries: Powering the EV Revolution
Countries across the globe are working towards a greener future and electric vehicles (EVs) are a key piece of the puzzle.
In fact, the EV revolution is well underway, rising from 17,000 electric cars in 2010 to 7.2 million in 2019—a 423x increase in less than a decade. At the same time, we often take for granted the variety of materials that make modern technology work. Going electric requires the use of strategic minerals, especially cobalt.
Today’s infographic comes to us from Fuse Cobalt and looks into how the cobalt in lithium batteries makes the difference for powerful and reliable battery technology.
Edging Over the Competition: The Lithium-Cobalt Combination
There are five primary lithium battery combinations for EVs, each with pros and cons:
- Lithium Nickel Cobalt Aluminum (NCA)
- Lithium Nickel Manganese Cobalt (NMC)
- Lithium Manganese Oxide (LMO)
- Lithium Titanate (LTO)
- Lithium Iron Phosphate (LFP)
From the plethora of lithium-ion battery compositions, EV manufacturers prefer the lithium-cobalt combination. As a result, NCA and NMC batteries are the most prevalent in EVs.
| NCA batteries | NMC batteries |
|---|---|
| Offer high specific energy and power Allow EVs to travel farther | Offer a similar caliber of performance |
| Use less cobalt, making them less expensive More prone to overheating | Use more cobalt, making them more expensive Higher overall safety |
| Commonly found in Tesla EVs | Commonly found in Nissan, Chevrolet, and BMW EVs |
The low energy density and power of the other batteries make them impractical for long-range EVs—and it’s partially due to the lack of cobalt.
Why Lithium-Cobalt?
When it comes to powering EVs, lithium-cobalt batteries are unmatched. Specific properties of cobalt make them stand out from the rest:
- High energy density
- Thermal stability
- High specific power
- Low self-discharge rate
- Low weight
- Recyclability
Not only do lithium-cobalt batteries allow EVs to travel farther, but they also improve safety and sustainability.
Cobalt: The Stable Battery Element
Cobalt’s high energy density allows batteries to pack more energy in smaller spaces, making them lightweight and powerful at the same time. In addition, its ability to withstand high temperatures increases the safety and reliability of EVs.
Furthermore, cobalt increases the longevity of batteries and remains highly recyclable, promoting a more sustainable battery supply chain.
Despite its advantages, EV manufacturers are making efforts to reduce the cobalt content of their batteries for various reasons associated with its supply chain:
- Cobalt is a by-product of nickel and copper mining, which makes it harder to obtain.
- Cobalt is expensive, at US$33,000/tonne—more than twice the price of nickel.
- The general public associates cobalt mining in the Congo with child labor, tough conditions, and corruption.
Although cobalt may be associated with unethical mining practices, it still remains essential to EV manufacturers—as demonstrated by Tesla’s agreement to buy 6,000 tonnes of cobalt annually from mining giant Glencore.
Combating Cobalt’s Ethical Concerns
EV manufacturers and miners have joined forces with organizations that are making efforts to alleviate the ethical issues associated with cobalt mining. These include:
- Fair Cobalt Alliance
- Responsible Minerals Initiative
- Responsible Cobalt Initiative
- Clean Cobalt Initiative
As these initiatives progress, we may see a future with ethically mined cobalt in EV batteries, including cobalt mined in more jurisdictions outside of the DRC.
For the time being, it’s interesting to see how lithium-cobalt batteries power up an EV.
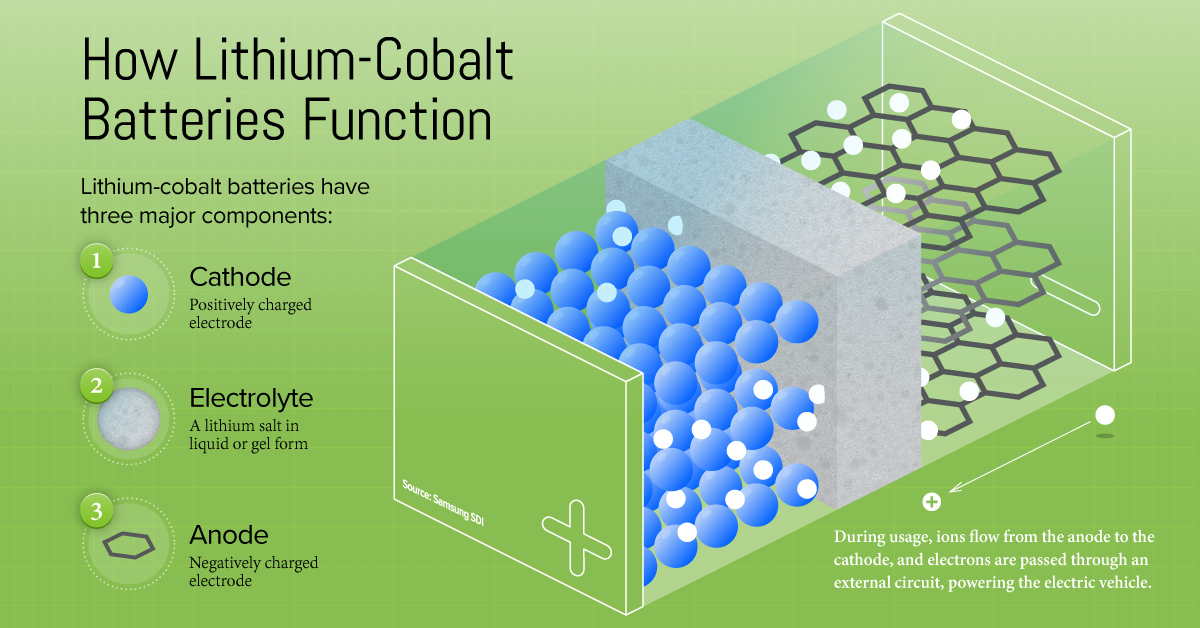
Breaking Down a Lithium-Cobalt Battery
Lithium-Cobalt batteries have three key components:
- The cathode is an electrode that carries a positive charge, and is made of lithium metal oxide combinations of cobalt, nickel, manganese, iron, and aluminum.
- The anode is an electrode that carries a negative charge, usually made of graphite.
- The electrolyte is a lithium salt in liquid or gel form, and allows the ions to flow from the cathode to the anode (and vice versa).
How it Works
When the battery is charged, lithium ions flow via the electrolyte from the cathode to the anode, where they are stored for usage. Simultaneously, electrons pass through an external circuit and are collected in the anode through a negative current collector.
When the battery is generating an electric current (i.e. discharging), the ions flow via the electrolyte from the anode to the cathode, and the electrons reverse direction along the external circuit, powering up the EV.
The composition of the cathode largely determines battery performance. For EV batteries, this is where the lithium-cobalt combination plays a crucial role.
The EV market could experience colossal growth over the next decade, but it faces several roadblocks. At present, EV charging infrastructure is expensive and not as convenient as the local gas station—and lithium-cobalt batteries could help overcome this obstacle.
Battery Storage: The Future of EV Charging Stations?
There are the two ways to charge an electric vehicle battery:
- Alternating Current (AC) chargers provide an alternating current, which periodically reverses direction.
- Direct Current (DC) fast chargers provide direct current that moves only in one direction.
But there’s a catch.
EV batteries can only store energy in the form of direct current. To charge an EV battery, the onboard charger must convert the alternating current from AC chargers into direct current, increasing charging times substantially.
Today, EV chargers are available in three different types:
| Type of Charger | Description | Max energy drawn per hour | Charge time (60-kWH EV battery) |
|---|---|---|---|
| Alternating Current (AC) Level 1 | Charge via a 120-volt AC plug | 1.4kW | 2,400 minutes |
| Alternating Current (AC) Level 2 | Charge via a 240-volt AC plug | 7.2kW | 500 minutes |
| Direct Current (DC) | Charge EVs rapidly, but are more expensive to install and use | 50-350kW | Range between 10-75 minutes |
Meanwhile, several roadblocks still discourage EV buyers, from the lack of charging infrastructure to long charging times.
Stationary battery storage could be the solution.
Stationary Battery Storage: Solving the EV Charging Enigma
Charged batteries can provide EVs with direct current without drawing power from the grid during times of high demand. This can significantly reduce the demand charges of electricity, which account for a large portion of a charging station’s electricity bill.
The highest rate of electricity usage at a particular time determines the demand charges, separate from the cost of actual energy consumed. In other words, demand charges can be astronomical at times when multiple vehicles are charged via power from the grid.
Stationary battery storage systems could be charged from the grid at times of low demand, and used to provide direct current to vehicles during times of high demand.
As a result, this could dramatically reduce charging times as well as the cost of electricity.
Enabling Stationary Battery Storage
Developing stationary battery storage systems on a large scale is expensive. Lithium-cobalt batteries could mitigate these costs through their recyclability.
Unless damaged beyond repair, recycling companies can refurbish lithium-cobalt battery packs for a second life as stationary storage systems.
Re-using batteries promotes a circular economy and reduces waste, pollution, and costs. Not only would this improve charging infrastructure, but it would also create a more sustainable supply chain for EV batteries.
Lithium-Cobalt Batteries: Here to Stay
Despite efforts to reduce the cobalt contents in batteries, the lithium-cobalt combination remains the optimal technology for EV batteries.
Growth is imminent in the EV market, and lithium-cobalt batteries could take center stage in improving both vehicle performance, and charging infrastructure.
-
 Sponsored3 years ago
Sponsored3 years agoMore Than Precious: Silver’s Role in the New Energy Era (Part 3 of 3)
Long known as a precious metal, silver in solar and EV technologies will redefine its role and importance to a greener economy.
-

 Sponsored7 years ago
Sponsored7 years agoThe History and Evolution of the Video Games Market
Everything from Pong to the rise of mobile gaming and AR/VR. Learn about the $100 billion video games market in this giant infographic.
-

 Sponsored8 years ago
Sponsored8 years agoThe Extraordinary Raw Materials in an iPhone 6s
Over 700 million iPhones have now been sold, but the iPhone would not exist if it were not for the raw materials that make the technology...
-

 Sponsored8 years ago
Sponsored8 years agoThe Industrial Internet, and How It’s Revolutionizing Mining
The convergence of the global industrial sector with big data and the internet of things, or the Industrial Internet, will revolutionize how mining works.